重度抑郁症 (MDD),俗称抑郁症,是一种使人衰弱的疾病,影响全球约3.8%的人口,其中5.0%是成年人,5.7%是60岁以上的人。MDD不同于常见的情绪变化和由于灰质和白质(包括额叶、海马体、颞叶、丘脑、纹状体和杏仁核)的细微变化而导致的短暂情绪反应。如果它以中等或严重的强度发生,它可能对一个人的整体健康有害。它会使一个人在个人、职业和社交生活中表现不佳而痛苦不堪。

一、简介
重度抑郁症 (MDD) 被认为是最常见的精神疾病,根据世界卫生组织 (WHO) 的说法,它是导致残疾的主要原因。情绪低落、对日常活动的兴趣下降、内疚、失去快乐、注意力难以集中、自卑、睡眠困难和食欲改变是 MDD 的一些症状。
这些问题可能会长期存在或反复出现,严重影响一个人进行日常活动的能力。在最坏的情况下,抑郁症会导致自杀念头。抑郁症与患其他严重疾病(如心血管疾病)的几率增加有关、中风、阿尔茨海默病、癫痫、糖尿病和癌症。抑郁症状在老年人中更常见,但这是由于与衰老相关的因素造成的,包括身体残疾、认知缺陷、社会经济缺陷和其他因素。难治性抑郁症 (TRD) 可由发育过程中持续暴露于环境压力源引起。几乎所有的抗抑郁药都以相同的方式起作用,并在整个生命周期内有效治疗严重的MDD。
然而,抗抑郁治疗有许多不良副作用,包括镇静、头痛、血压下降、失眠、体重增加、消化不良、情绪激动、口干、腹泻和性功能障碍。这通常会导致患者依从性差,导致抑郁症状复发和更高的自杀风险。
2. 抑郁症的神经化学:单胺假说
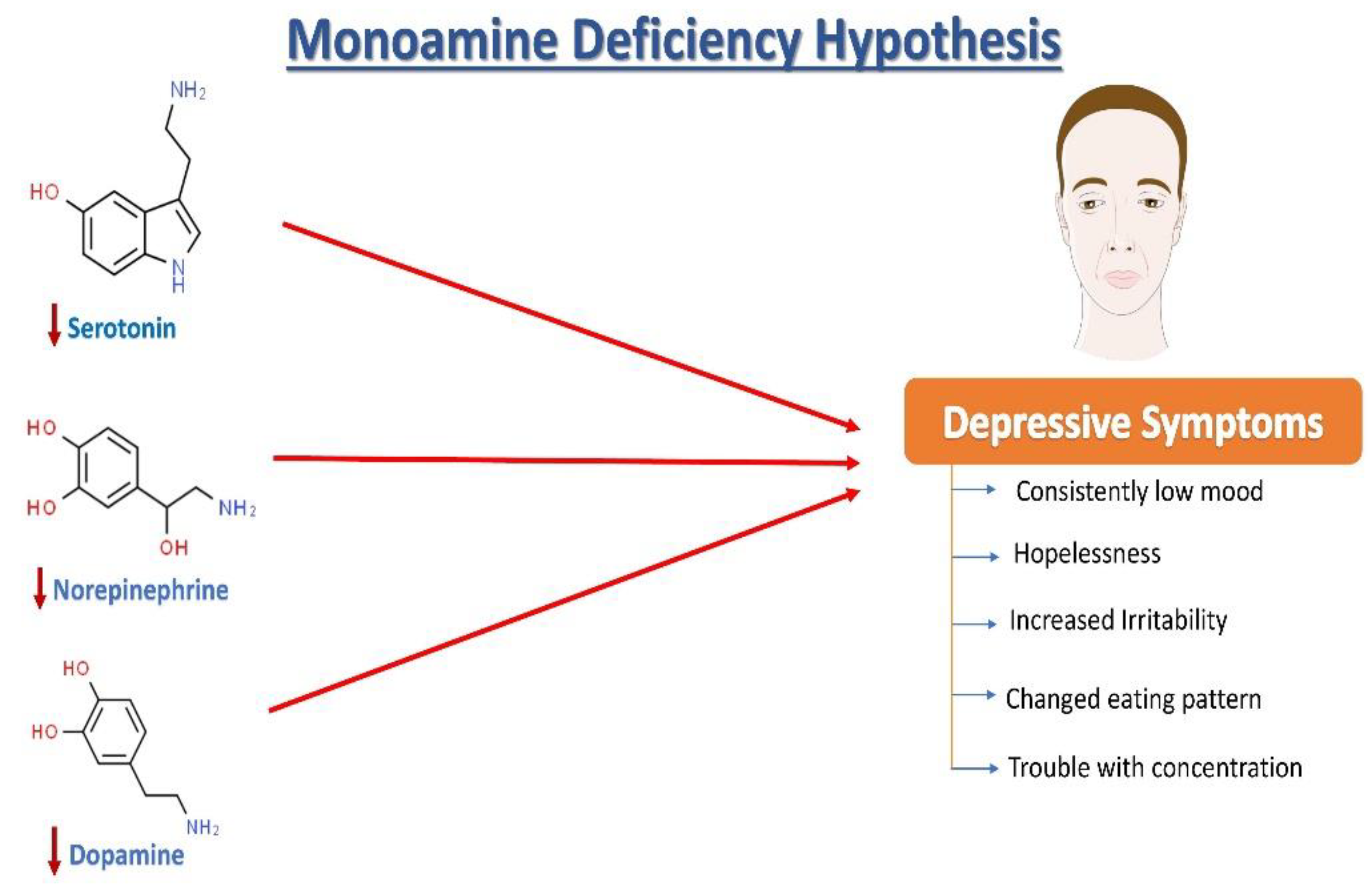
去甲肾上腺素 (NE)、血清素(5-羟色胺,5HT)和多巴胺 (DA) 失调与抑郁症的病理变化有关(图1)。根据抑郁症的单胺假说,NE、5HT和DA同步发挥作用,调节情绪和情绪。在情绪低落时,观察到这三种单胺的失调,以及细胞外5HT水平低于平均水平。据报道,与年龄匹配的对照组相比,抑郁症患者的尿液、血液和脑脊液 (CSF) 中的单胺类和代谢物含量较低。
3. 抑郁症的生长因子
有几种与抑郁症状相关的生物学因素,如中所示。与抑郁行为相关的神经回路中的突触可塑性受脑源性神经营养因子(BDNF)调节。有趣的是,压力引起的大脑结构和突触可塑性损伤可能会被BDNF上调逆转,从而导致认知的灵活性和适应可能刺激抑郁发作的环境变化的能力提高。根据目前的研究,在抑郁症受试者中,血液中的BDNF水平较低,并且随着抗抑郁治疗的增加而增加,如图2。研究表明,应激诱导的表观遗传变化可导致抑郁症。对MDD颞叶结构研究的两项荟萃分析表明,复发性抑郁症患者的海马体较小。此外,无论使用何种药物,升高的BDNF血浆水平都与更好的治疗结果有关。
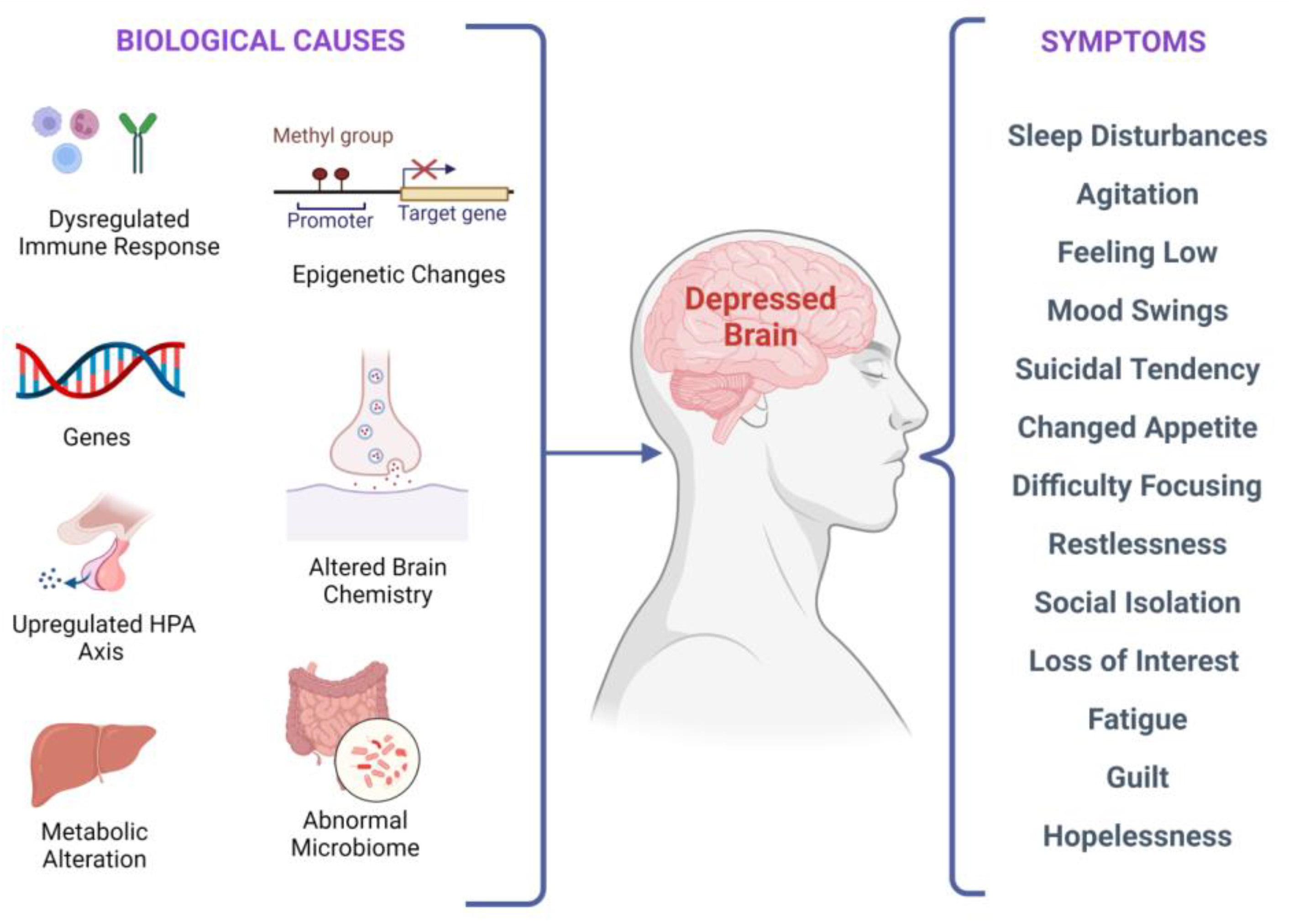
在分子、遗传、表观遗传、细胞和系统水平上有多种生物学原因。这些原因会导致临床抑郁症,并且可能会出现多种症状,这些症状可能因人而异。
4. 神经干细胞与抑郁症
近年来,神经干细胞移植引起了人们的兴趣,大量发表的文献阐明成人大脑维持多能NSCs,这与大脑的旧教条形成鲜明对比,大脑通常是不变的和静止的器官,缺乏再生的灵活性。凭借其最普遍接受的显着特征,NSCs也被归于所谓的组织
干细胞 ,具有在特定条件下保持未分化而没有概述表型的能力、分裂和增殖(自我更新)的能力,以及在神经发生开始时分化成神经元、少突胶质细胞和星形胶质细胞等后代的能力。它们是在成年哺乳动物大脑的“神经源性”区域(例如海马体)中发现的独特类型的感受态细胞、脑室下区和神经结构,并可能自发地和响应局部信号感应产生神经元。神经发生 (NG) 被认为需要一组明确的信号线索,以通过周围环境在空间和时间上非常协调的方式传递给神经源性细胞,以激活干细胞或祖细胞以发育新的神经元,此外,众所周知的调制器,损伤被认为足以激活神经发生。BDNF 的表达也会刺激神经发生。NSC通常是从成人脑组织中提取的,包括死后脑组织,并成为增加或恢复受中枢神经系统相关疾病影响的脑组织质量和功能的重要候选者。NSC在体外进行克隆扩增、基因操作或刺激以转化CNS细胞谱系。了解成人神经发生的调节方式需要大量工作。
生长因子、递质、酶、组织激素、神经调节剂和抗体预计会被激活的细胞分泌到局部组织环境中,从而引发所需的组织反应。在受损的神经元和神经胶质网络中,新赋能的细胞及其后代可以作为功能增强剂和支架“修复剂”发挥作用。这些特性导致创伤和灌注问题(如中风 、局部缺血或神经退行性病)疗法的发明取得实质性进展。
不出所料,NSCs在精神卫生保健方面的前景正在引起激烈争论。许多精神疾病可能具有遗传变异以及大多数未知的特定细胞和解剖相关性。
在抑郁症中,海马体中经常会出现神经发生减少。这进一步意味着神经发生缺陷可能导致与抑郁症相关的症状,而增强的神经发生可以介导抗抑郁作用并缓解症状。
然而,在建立这种双向概念的完全合法性之前,必须首先调和关于神经发生在缓解抑郁症中的作用的各种相互矛盾的报告。成人海马神经发生的激活导致神经体细胞后代转化为成熟的CNS神经元。然后,这些中枢神经系统神经元获得功能和形态特性,以整合到现有的神经网络或替换其他各种已经死亡的脑细胞。
参考资料:
Maj, M. When Does Depression Become a Mental Disorder? Br. J. Psychiatry 2011, 199, 85–86.
Lang, U.E.; Borgwardt, S. Molecular Mechanisms of Depression: Perspectives on New Treatment Strategies. Cell Physiol. Biochem. 2013, 31, 761–777.
Ramasubbu, R.; Patten, S.B. Effect of Depression on Stroke Morbidity and Mortality. Can. J. Psychiatry 2003, 48, 250–257.
Van der Kooy, K.; van Hout, H.; Marwijk, H.; Marten, H.; Stehouwer, C.; Beekman, A. Depression and the Risk for Cardiovascular Diseases: Systematic Review and Meta Analysis. Int. J. Geriat. Psychiatry 2007, 22, 613–626.
Green, R.C.; Cupples, L.A.; Kurz, A.; Auerbach, S.; Go, R.; Sadovnick, D.; Duara, R.; Kukull, W.A.; Chui, H.; Edeki, T.; et al. Depression as a Risk Factor for Alzheimer Disease: The MIRAGE Study. Arch. Neurol. 2003, 60, 753.
Hesdorffer, D.C.; Hauser, W.A.; Annegers, J.F.; Cascino, G. Major Depression Is a Risk Factor for Seizures in Older Adults. Ann. Neurol. 2000, 47, 246–249.
Nouwen, A.; Lloyd, C.E.; Pouwer, F. Depression and Type 2 Diabetes Over the Lifespan: A Meta-Analysis. Diabetes Care 2009, 32, e56.
Penninx, B.W.J.H.; Guralnik, J.M.; Havlik, R.J.; Pahor, M.; Ferrucci, L.; Cerhan, J.R.; Wallace, R.B. Chronically Depressed Mood and Cancer Risk in Older Persons. JNCI J. Natl. Cancer Inst. 1998, 90, 1888–1893.
Kaur, J.; Ghosh, S.; Singh, P.; Dwivedi, A.K.; Sahani, A.K.; Sinha, J.K. Cervical Spinal Lesion, Completeness of Injury, Stress, and Depression Reduce the Efficiency of Mental Imagery in People With Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2022, 101, 513–519.
Maniam, J.; Antoniadis, C.P.; Youngson, N.A.; Sinha, J.K.; Morris, M.J. Sugar Consumption Produces Effects Similar to Early Life Stress Exposure on Hippocampal Markers of Neurogenesis and Stress Response. Front. Mol. Neurosci. 2015, 8, 86.
Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917.
Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J. Treatment Resistant Depression: A Multi-Scale, Systems Biology Approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288.
Salzman, C.; Wong, E.; Wright, B.C. Drug and ECT Treatment of Depression in the Elderly, 1996–2001: A Literature Review. Biol. Psychiatry 2002, 52, 265–284.
Keller, M.B.; Hirschfeld, R.M.A.; Demyttenaere, K.; Baldwin, D.S. Optimizing Outcomes in Depression: Focus on Antidepressant Compliance. Int. Clin. Psychopharmacol. 2002, 17, 265–271.
Nestler, E.J.; Carlezon, W.A. The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 2006, 59, 1151–1159.
Roy, A. Cerebrospinal Fluid Monoamine Metabolites and Suicidal Behavior in Depressed Patients: A 5-Year Follow-up Study. Arch. Gen. Psychiatry 1989, 46, 609.
Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced Levels of Norepinephrine Transporters in the Locus Coeruleus in Major Depression. J. Neurosci. 1997, 17, 8451–8458.
Marshe, V.S.; Maciukiewicz, M.; Rej, S.; Tiwari, A.K.; Sibille, E.; Blumberger, D.M.; Karp, J.F.; Lenze, E.J.; Reynolds, C.F.; Kennedy, J.L.; et al. Norepinephrine Transporter Gene Variants and Remission From Depression With Venlafaxine Treatment in Older Adults. Am. J. Psychiatry 2017, 174, 468–475.
Dunn, A.J. Effects of Cytokines and Infections on Brain Neurochemistry. Clin. Neurosci. Res. 2006, 6, 52–68.
Anacker, C.; Cattaneo, A.; Musaelyan, K.; Zunszain, P.A.; Horowitz, M.; Molteni, R.; Luoni, A.; Calabrese, F.; Tansey, K.; Gennarelli, M.; et al. Role for the Kinase SGK1 in Stress, Depression, and Glucocorticoid Effects on Hippocampal Neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 8708–8713.
Jokinen, J.; Nordström, A.-L.; Nordström, P. The Relationship Between CSF HVA/5-HIAA Ratio and Suicide Intent in Suicide Attempters. Arch. Suicide Res. 2007, 11, 187–192.
Pizzagalli, D.A.; Berretta, S.; Wooten, D.; Goer, F.; Pilobello, K.T.; Kumar, P.; Murray, L.; Beltzer, M.; Boyer-Boiteau, A.; Alpert, N.; et al. Assessment of Striatal Dopamine Transporter Binding in Individuals With Major Depressive Disorder: In Vivo Positron Emission Tomography and Postmortem Evidence. JAMA Psychiatry 2019, 76, 854.
Cassano, P.; Lattanzi, L.; Fava, M.; Navari, S.; Battistini, G.; Abelli, M.; Cassano, G.B. Ropinirole in Treatment-Resistant Depression: A 16-Week Pilot Study. Can. J. Psychiatry 2005, 50, 357–360.
Descarries, L.; Watkins, K.C.; Garcia, S.; Beaudet, A. The Serotonin Neurons in Nucleus Raphe Dorsalis of Adult Rat: A Light and Electron Microscope Radioautographic Study. J. Comp. Neurol. 1982, 207, 239–254.
Bunin, M.A.; Wightman, R.M. Quantitative Evaluation of 5-Hydroxytryptamine (Serotonin) Neuronal Release and Uptake: An Investigation of Extrasynaptic Transmission. J. Neurosci. 1998, 18, 4854–4860.
Steinbusch, H.W.M. Distribution of Serotonin-Immunoreactivity in the Central Nervous System of the Rat—Cell Bodies and Terminals. Neuroscience 1981, 6, 557–618.
Mann, J. Role of the Serotonergic System in the Pathogenesis of Major Depression and Suicidal Behavior. Neuropsychopharmacology 1999, 21, 99S–105S.
Chaouloff, F. Serotonin and Stress. Neuropsychopharmacology 1999, 21, 28S–32S.
Andrews, P.W.; Bharwani, A.; Lee, K.R.; Fox, M.; Thomson, J.A. Is Serotonin an Upper or a Downer? The Evolution of the Serotonergic System and Its Role in Depression and the Antidepressant Response. Neurosci. Biobehav. Rev. 2015, 51, 164–188.
Bot, M.; Chan, M.K.; Jansen, R.; Lamers, F.; Vogelzangs, N.; Steiner, J.; Leweke, F.M.; Rothermundt, M.; Cooper, J.; Bahn, S.; et al. Serum Proteomic Profiling of Major Depressive Disorder. Transl. Psychiatry 2015, 5, e599.
Quintana, J. Platelet Serotonin and Plasma Tryptophan Decreases in Endogenous Depression. Clinical, Therapeutic, and Biological Correlations. J. Affect. Disord. 1992, 24, 55–62.
Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, Epigenetics and Depression: A Systematic Review. Neurosci. Biobehav. Rev. 2019, 102, 139–152.
Ghosh, S.; Sinha, J.K.; Raghunath, M. “Obesageing”: Linking Obesity & Ageing. Indian J. Med. Res. 2019, 149, 610–615.
Campbell, S.; MacQueen, G. An Update on Regional Brain Volume Differences Associated with Mood Disorders. Curr. Opin. Psychiatry 2006, 19, 25–33.
Videbech, P. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Am. J. Psychiatry 2004, 161, 1957–1966.
Mishra, P.; Mittal, A.K.; Kalonia, H.; Madan, S.; Ghosh, S.; Sinha, J.K.; Rajput, S.K. SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan. Curr. Neuropharmacol. 2021, 19, 1019–1037.
Moroi, K.; Sato, T. Comparison between Procaine and Isocarboxazid Metabolism in Vitro by a Liver Microsomal Amidase-Esterase. Biochem. Pharmacol. 1975, 24, 1517–1521.
Pittenger, C.; Duman, R.S. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 2008, 33, 88–109.
Aydemir, O.; Deveci, A.; Taneli, F. The Effect of Chronic Antidepressant Treatment on Serum Brain-Derived Neurotrophic Factor Levels in Depressed Patients: A Preliminary Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265.
Ricken, R.; Adli, M.; Lange, C.; Krusche, E.; Stamm, T.J.; Gaus, S.; Koehler, S.; Nase, S.; Bschor, T.; Richter, C.; et al. Brain-Derived Neurotrophic Factor Serum Concentrations in Acute Depressive Patients Increase During Lithium Augmentation of Antidepressants. J. Clin. Psychopharmacol. 2013, 33, 806–809.
Bauer, M.; Adli, M.; Bschor, T.; Pilhatsch, M.; Pfennig, A.; Sasse, J.; Schmid, R.; Lewitzka, U. Lithium’s Emerging Role in the Treatment of Refractory Major Depressive Episodes: Augmentation of Antidepressants. Neuropsychobiology 2010, 62, 36–42.
Coradduzza, D.; Garroni, G.; Congiargiu, A.; Balzano, F.; Cruciani, S.; Sedda, S.; Nivoli, A.; Maioli, M. MicroRNAs, Stem Cells in Bipolar Disorder, and Lithium Therapeutic Approach. Int. J. Mol. Sci. 2022, 23, 10489.
Mondal, A.C.; Fatima, M. Direct and Indirect Evidences of BDNF and NGF as Key Modulators in Depression: Role of Antidepressants Treatment. Int. J. Neurosci. 2019, 129, 283–296.
de Miranda, A.S.; de Barros, J.L.V.M.; Teixeira, A.L. Is Neurotrophin-3 (NT-3): A Potential Therapeutic Target for Depression and Anxiety? Expert Opin. Ther. Targets 2020, 24, 1225–1238.
Diniz, B.S.; Teixeira, A.L.; Miranda, A.S.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Circulating Glial-Derived Neurotrophic Factor Is Reduced in Late-Life Depression. J. Psychiatr. Res. 2012, 46, 135–139.
Evans, S.J.; Choudary, P.V.; Neal, C.R.; Li, J.Z.; Vawter, M.P.; Tomita, H.; Lopez, J.F.; Thompson, R.C.; Meng, F.; Stead, J.D.; et al. Dysregulation of the Fibroblast Growth Factor System in Major Depression. Proc. Natl. Acad. Sci. USA 2004, 101, 15506–15511.
Beaulieu, J.-M. A Role for Akt and Glycogen Synthase Kinase-3 as Integrators of Dopamine and Serotonin Neurotransmission in Mental Health. J. Psychiatry Neurosci. 2012, 37, 7–16.
Miskowiak, K.W.; Vinberg, M.; Harmer, C.J.; Ehrenreich, H.; Knudsen, G.M.; Macoveanu, J.; Hansen, A.R.; Paulson, O.B.; Siebner, H.R.; Kessing, L.V. Effects of Erythropoietin on Depressive Symptoms and Neurocognitive Deficits in Depression and Bipolar Disorder. Trials 2010, 11, 97.
Eden Evins, A.; Demopulos, C.; Yovel, I.; Culhane, M.; Ogutha, J.; Grandin, L.D.; Nierenberg, A.A.; Sachs, G.S. Inositol Augmentation of Lithium or Valproate for Bipolar Depression. Bipolar Disord. 2006, 8, 168–174.
Cattaneo, A.; Sesta, A.; Calabrese, F.; Nielsen, G.; Riva, M.A.; Gennarelli, M. The Expression of VGF Is Reduced in Leukocytes of Depressed Patients and It Is Restored by Effective Antidepressant Treatment. Neuropsychopharmacology 2010, 35, 1423–1428.
Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848.
Kukekov, V.G.; Laywell, E.D.; Suslov, O.; Davies, K.; Scheffler, B.; Thomas, L.B.; O’Brien, T.F.; Kusakabe, M.; Steindler, D.A. Multipotent Stem/Progenitor Cells with Similar Properties Arise from Two Neurogenic Regions of Adult Human Brain. Exp. Neurol. 1999, 156, 333–344.
Alvarez-Buylla, A.; García-Verdugo, J.M. Neurogenesis in Adult Subventricular Zone. J. Neurosci. 2002, 22, 629–634.
Liu, Z.; Martin, L.J. Olfactory Bulb Core Is a Rich Source of Neural Progenitor and Stem Cells in Adult Rodent and Human. J. Comp. Neurol. 2003, 459, 368–391.
Kempermann, G. Regulation of Adult Hippocampal Neurogenesis—Implications for Novel Theories of Major Depression 1: Regulation of Adult Hippocampal Neurogenesis. Bipolar Disord. 2002, 4, 17–33.
Mansoor, A.K.; Thomas, S.; Sinha, J.K.; Alladi, P.A.; Ravi, V.; Raju, T.R. Olfactory tract transection reveals robust tissue-level plasticity by cellular numbers and neurotrophic factor expression in olfactory bulb. Indian J. Exp. Biol. 2012, 50, 765–770.
Feldmann, R.E.; Mattern, R. The Human Brain and Its Neural Stem Cells Postmortem: From Dead Brains to Live Therapy. Int. J. Leg. Med. 2006, 120, 201–211.
Lindvall, O.; Kokaia, Z. Recovery and Rehabilitation in Stroke: Stem Cells. Stroke 2004, 35, 2691–2694.
Sachdeva, P.; Ghosh, S.; Ghosh, S.; Han, S.; Banerjee, J.; Bhaskar, R.; Sinha, J.K. Childhood Obesity: A Potential Key Factor in the Development of Glioblastoma Multiforme. Life 2022, 12, 1673.
Ghosh, S.; Manchala, S.; Raghunath, M.; Sharma, G.; Singh, A.K.; Sinha, J.K. Role of Phytomolecules in the Treatment of Obesity: Targets, Mechanisms and Limitations. Curr. Top. Med. Chem. 2021, 21, 863–877.
Goldman, S. Stem and Progenitor Cell–Based Therapy of the Human Central Nervous System. Nat. Biotechnol. 2005, 23, 862–871.
Lipska, B.K. Using Animal Models to Test a Neurodevelopmental Hypothesis of Schizophrenia. J. Psychiatry Neurosci. 2004, 29, 282–286.
Feldmann, R.E.; Sawa, A.; Seidler, G.H. Causality of Stem Cell Based Neurogenesis and Depression—To Be or Not to Be, Is That the Question? J. Psychiatr. Res. 2007, 41, 713–723.
Zhao, C. Distinct Morphological Stages of Dentate Granule Neuron Maturation in the Adult Mouse Hippocampus. J. Neurosci. 2006, 26, 3–11.
免责说明:本文仅用于传播科普知识,分享行业观点,不构成任何临床诊断建议!杭吉干细胞不保证信息的准确性和完整性。所发布的信息不能替代医生或药剂师的专业建议。如有版权等疑问,请随时联系我。




 浙公网安备 33010202002429号
浙公网安备 33010202002429号 官方微信公众号
官方微信公众号